

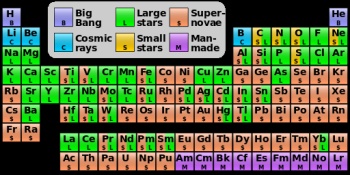
If you look up any chemical element at Wikipedia, the article will likely tell you how it was synthesized by stars, produced by supernovae, spallation, or radioactive decay. (Spallation is the splitting atoms by high energy cosmic rays). This reasoning generally explains the relative abundance of elements both here on Earth and in space. After the dawn of the nuclear age Margaret Burbidge, Geoffrey Burbidge, William Fowler, and Fred Hoyle came to realize that the abundance of elements and the life cycle of stars could be explained by stellar nucleosythesis. Their 1957 landmark work Synthesis of Elements in Stars stands to this day as a pillar of modern astronomy.
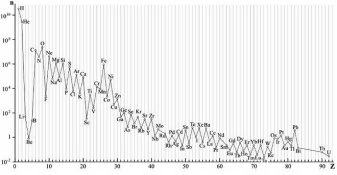

I first learned about nucleosynthesis and elemental abundance many years ago from Isaac Asmimov. His books taught me astronomy as far back as 1969. In one of his books he mused about life's bottleneck element, Phosphorous; and about two of the rarest naturally occurring elements -- Francium and Astatine. Francium's two naturally occurring isotopes are Fr-221 and Fr-223 with half lives of 4.8 minutes and 22 minutes respectively. A bulk collection of Francium atoms has never been observed thus it's chemical properties are mere speculation. It's estimated that about one ounce of this element exists in the entire Earth's crust as transient decay products of Uranium and Thorium. The most common, naturally occuring isotope of Astatine has a half life of only 32 milliseconds and about 100 milligrams is expected to exist in the Earth's crust at any given moment.
There are four primary radioactive decay series. These heaviest elements were created during supernova explosions and dispersed into the hydrogen cloud that eventually collapsed to form the sun and solar system. The first series really begins with Thorium-232 as it is the first element in the series with a half-life on the order of the age of the solar system. Plutonium 244 with an 80 million year half-life would have been reduced to 5 billionths of a billionth of its original amount after 57 half-lives since the solar system began. The Neptunium decay series has all decayed to Bismuth-209 as 2000 half-lives have passed for Neptunium-237, its longest lived element. The Radium series starts with Uranium-238, and the Actinium series starts with Uranium-235.
|
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Measuring the relative abundances of radioactive elements and their final decay products (Lead 206,207, and 208) allows us to determine the age of the Earth and solar system. By measuring the ratio of decay products from different decay series, scientists are able to construct multiple time-lines that intersect at their beginning. Clair Cameron Patterson of Mitchellville, Iowa and Grinell College, studied meteorites using a mass spectrometer at Argonne National Laboratory. In 1956 he deduced the age of the Earth to be 4.55 billion years and this value has stood the test of time. Incidentally, Clair campaigned to end the use of leaded gasoline which ultimately spared millions from low levels of lead poisoning thus ultimately raising the average IQ of the industrialized world. Dr. Patterson's work to ban tetraethyl lead from gasoline was strongly opposed by the Ethyl corporation at the time, but science won in the end. It's a prime example of how work in one field of science had a significant human impact in a seemingly unrelated field. Iowan Dr. James Hansen is currently on a strikingly similar journey which began with the study of the atmosphere of Venus and led to a campaign against fossil fuel corporations regarding climate change.

The neptunium decay series was not known at the time Dr. Patterson did his work, as it was only recently discovered. Although primordial Neptunium has disappeared from Earth, it might surprise you to learn you probably have a small amount of this stuff in your home. Your smoke detector is powered in part by Americium 241 which was created in nuclear reactors as a radioactive waste product. About 1 μg is used as a source of alpha particles which ionizes gas to create a small current which can be blocked by smoke, setting off the detector. A microgram of Am-241 (241/Avogadro's number) would contain about 2.5 X 1015 atoms with a half-life of 433 years. If you've had your smoke detector for 10 years, your smoke detector now contains 80 trillion atoms of Neptunium-237. And it may even contain up to 10 atoms of Bismuth-209, which means it also briefly contained Francium and Astatine.
