

Neutrinos, Big Numbers, and Four Forces
Astronomy is full of atronomically big numbers and big ideas. For example, the nearest star, Proxima Centauri, is 4.24. light years away. A light year is 5.88 trillion miles thus Proxima Centauri is 24,900,000,000,000 miles away. We can short hand such big numbers with exponential notation -- 24.9 X 1012. It can be a bit difficult to conceptualize a number that big, but with the help of a football field we shall take a stab at it. We'll kill several birds with one stone by using our field to construct a scale model of an atom. The model of an atom below is highly schematic -- definitely not to scale.


Consider how much an atom is empty space! The "solid" parts are only one part in a quadrillion. If we shrink the atom back to normal size then there are a million of them across the diameter of a human hair. But shrinking them doesn't alter the proportions of the nucleus to empty space. If we were to eliminate the empty space in you, then the solid part of you would fit into a single cell. But if you are mostly empty space, then why do you seem to be solid? Why does everything around us seem to be solid? Why can't I pass my hand through walls? The answer is the electromagnetic force -- one of the four fundamental forces in nature. The protons are positively charged and the electrons are negatively charged. Like charges repel each other. It is the EM force that is responsible for nearly all of the phenomena around us. It is responsible for chemical bonding and reactions, it is source of light, sound, touch, taste, and smell. And it makes us appear to be solid.
It is quite a strong force compared to the other fundamental force we are all familiar with -- gravity. But one thing about the EM force is that is is almost always in balance. Gravity and the EM force are similar in that they extend over long ranges, but gravity can never be balanced. There is no anti-gravity force. The more of something you have, the more gravity it has. Now consider a familiar experience -- picking up a metal object with a magnet. Magnetism is a very tiny residual EM force caused by the atoms in a material having their spins aligned. The object being picked up has a force acting on it to resist being picked up -- gravity. And that gravity is being produced by the sum of all the particle in the entire planet. Gravity is a very, very weak force compared to EM. In fact it is 1036 times weaker than the electromagnetic force. Oops! That's a number too big to vizualize with our football field! We come up 21 zeroes short!
There are two less familiar forces, and both are rather important in astronomy. They are the strong nuclear force and the weak nuclear force. Both act over very short ranges. The strong nuclear force holds protons and neutrons together in the nucleus and it has a range of about twice the diameter of the nucleus. Two protons strongly repel each other due to their like charges. However, if the the temperature and pressure is high enough, such as in the center of a star, they forced close enough that the nuclear force takes over which is 100 times stronger than the EM force. This is nuclear fusion and it's what powers the sun and stars. Fusing hydrogen to helium results in a loss of a small amount of mass which is turned into energy.
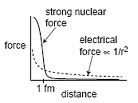

The weak nuclear force has a range of about 1% of a nuclear diameter. Each fusion reaction produces neutrinos which are nearly massless and only interact via the weak nuclear force. Therefore, they can travel through atoms unimpeded and rarely interact with nucleons. To a neutrino, the atom is truly empty space -- even more so than our football field model would suggest. There are 65 billion neutrinos zipping through your thumbnail every second from the nuclear reactions at the center of the sun. Huge neutrino detectors have been built underground using tons of material as an interaction medium. Until recently, these detectors registered neutrino strikes less than once a day. Changes in reactions in the sun's ultra-dense core can take 100 millenia to filter to the sun's surface via the EM force and then another eight minutes to reach earth. Neutrinos from the nuclear reactions in the sun's core reach us in eight minutes.
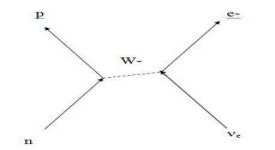
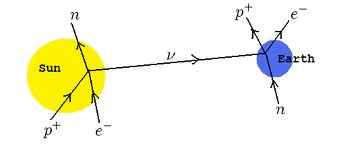
There are several neutrino detectors around the world, all buried deep underground to eliminate background radiation. Most were designed with the aim of observing nuclear reactions in the sun's core. But on February 23, 1987, the detectors observed a supernova in another galaxy! That will be our next story.